
1
Symbols to be used on
labelling (ISO 15223)
&
Information to be provided by
the manufacturer (ISO 20417)
Peter Bowness PhD
Technical Team Manager - Medicinal & Biologics
Copyright © 2020 BSI. All rights reserved

2
Symbols to be used on labelling (ISO 15223) & Information to be
provided by the manufacturer (ISO 20417)
• ISO 15223 – changes
• ISO 15223 – new
• ISO 15223 current status
• ISO 20417 – new
• ISO 20417 overview of contents
• ISO 20417 current status
Copyright © 2020 BSI. All rights reserved

3
Symbols to be used on labelling (ISO 15223)
• Introduces some new symbols
• Updated text for other symbols
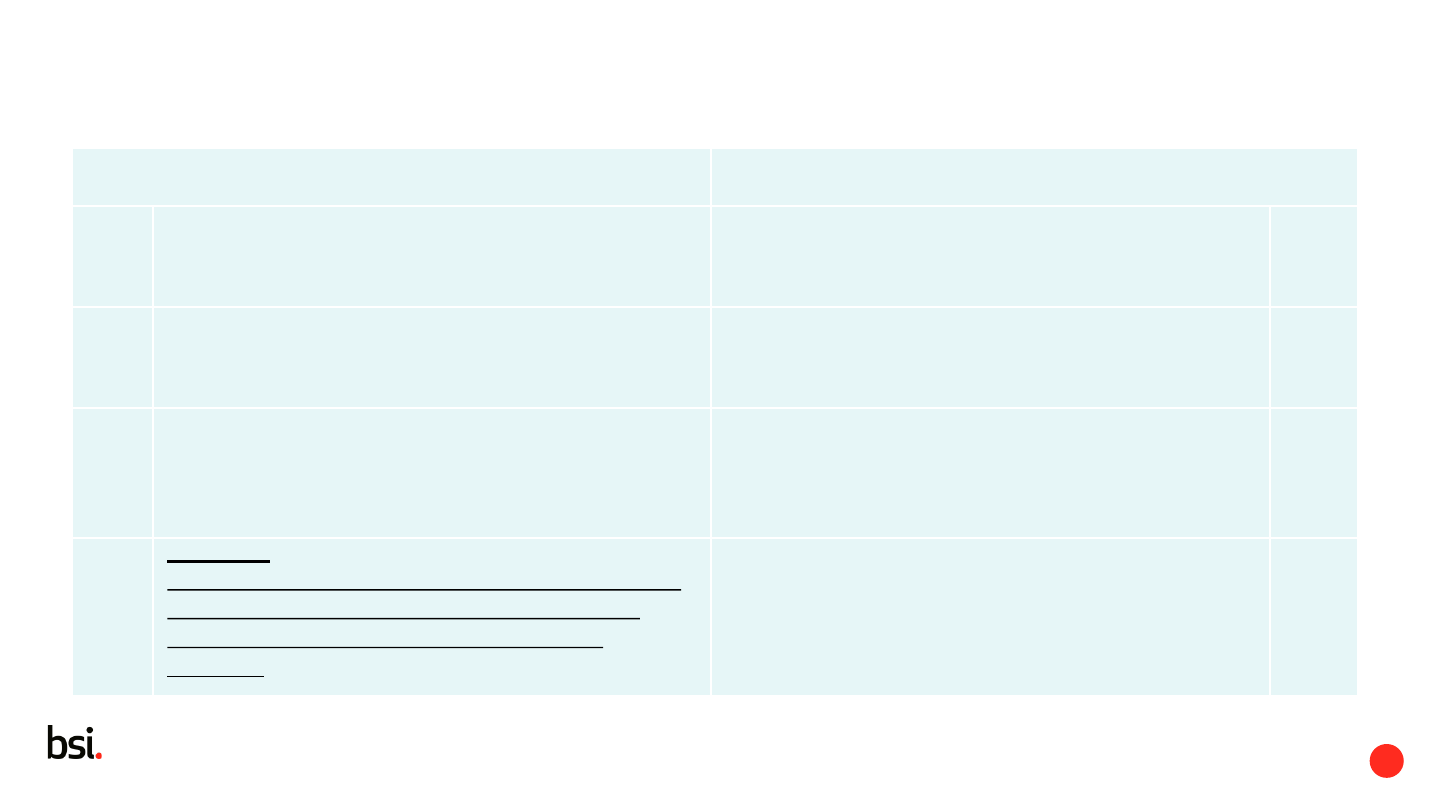
Safety Sign (ISO 7010) Symbol (ISO 15223)
sign giving a general safety message, obtained
by a combination of a colour and geometric
shape and which, by the addition of a graphical
symbol, gives a particular safety message
graphical representation appearing on the label
and/or associated documentation of a medical
device that communicates characteristic
information without the need for the supplier or
receiver of the information to have knowledge of
the language of a particular nation or people
Copyright © 2020 BSI. All rights reserved
4
Symbols to be used on labelling (ISO 15223)
• New symbols required by EU Regulations
• Updates to add clarity to existing symbols and definitions
• Draft status – final draft is in progress
Copyright © 2020 BSI. All rights reserved
5
Symbols to be used on labelling (ISO 15223)
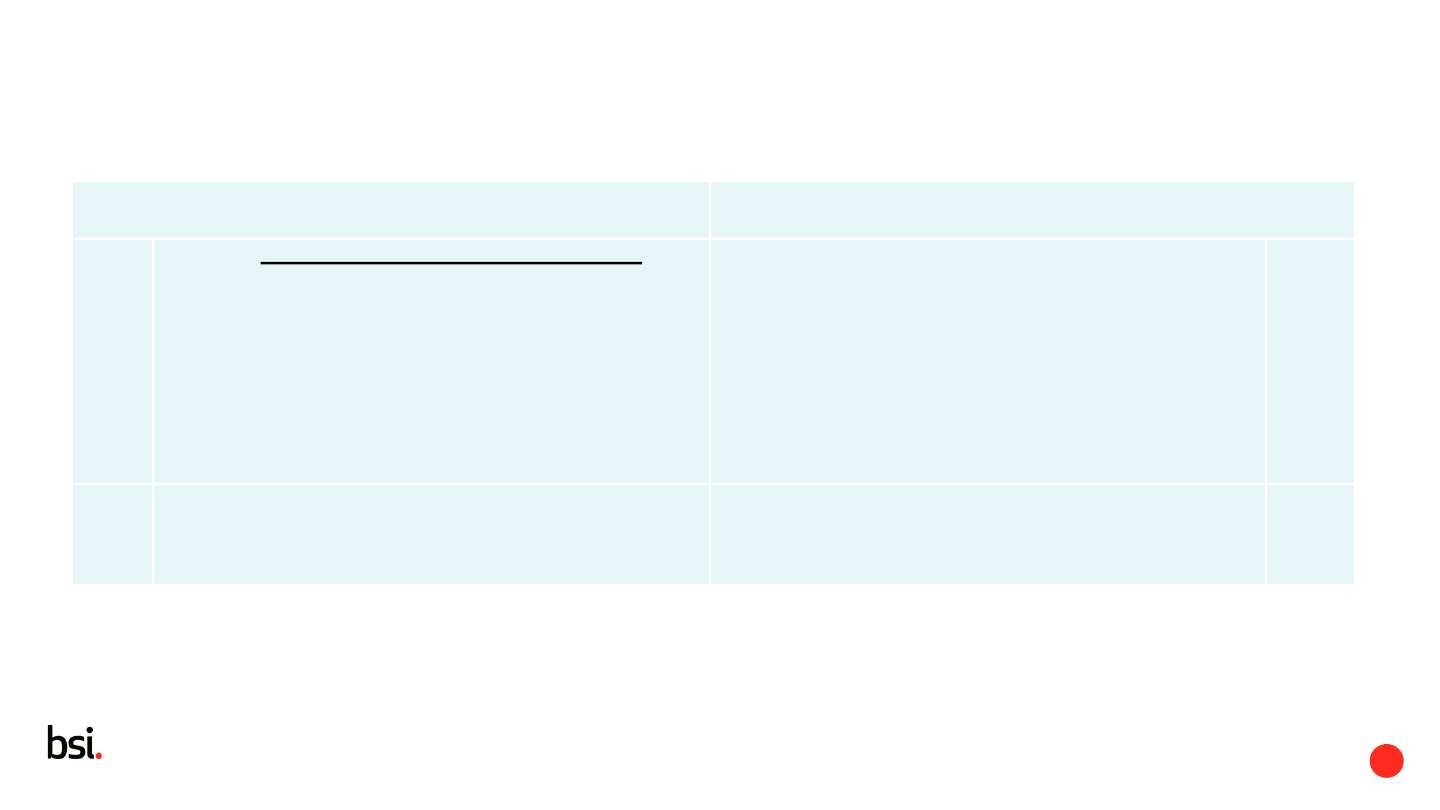
EN ISO 15223-1:2016 ISO/DIS 15223-1
3.1 characteristic information
information that represents the property or
properties of a symbol
No change 3.1
3.2 description
normative text which defines the purpose,
application and use of the symbol
No change 3.2
3.3 label
written, printed or graphic information provided
upon the medical device itself
label
written, printed or graphic information marked on
the item itself, or on the packaging of each item,
or on the packaging of multiple items
3.3
3.4
labelling
information supplied by the manufacturer that is
provided for, associated with, or affixed to, a
medical device or any of its containers or
wrappers
Copyright © 2020 BSI. All rights reserved
6
Symbols to be used on labelling (ISO 15223)
EN ISO 15223-1:2016 ISO/DIS 15223-1
3.5
symbol used in medical device labelling
graphical representation appearing on the label
(3.3) and/or associated documentation of a
medical device that communicates characteristic
information (3.1) without the need for the
supplier or receiver of the information to have
knowledge of the language of a particular nation
or people
symbol
graphical representation appearing on the label
(3.3) and/or associated documentation of a
medical device that communicates characteristic
information (3.1) without the need for the
supplier or receiver of the information to have
knowledge of the language of a particular nation
or people
3.4
3.6 title
unique name by which a graphical symbol is
identified and spoken of
title
unique name by which a graphical symbol is
identified and referenced
3.5
Copyright © 2020 BSI. All rights reserved
7
5.1.1
23.2(c)
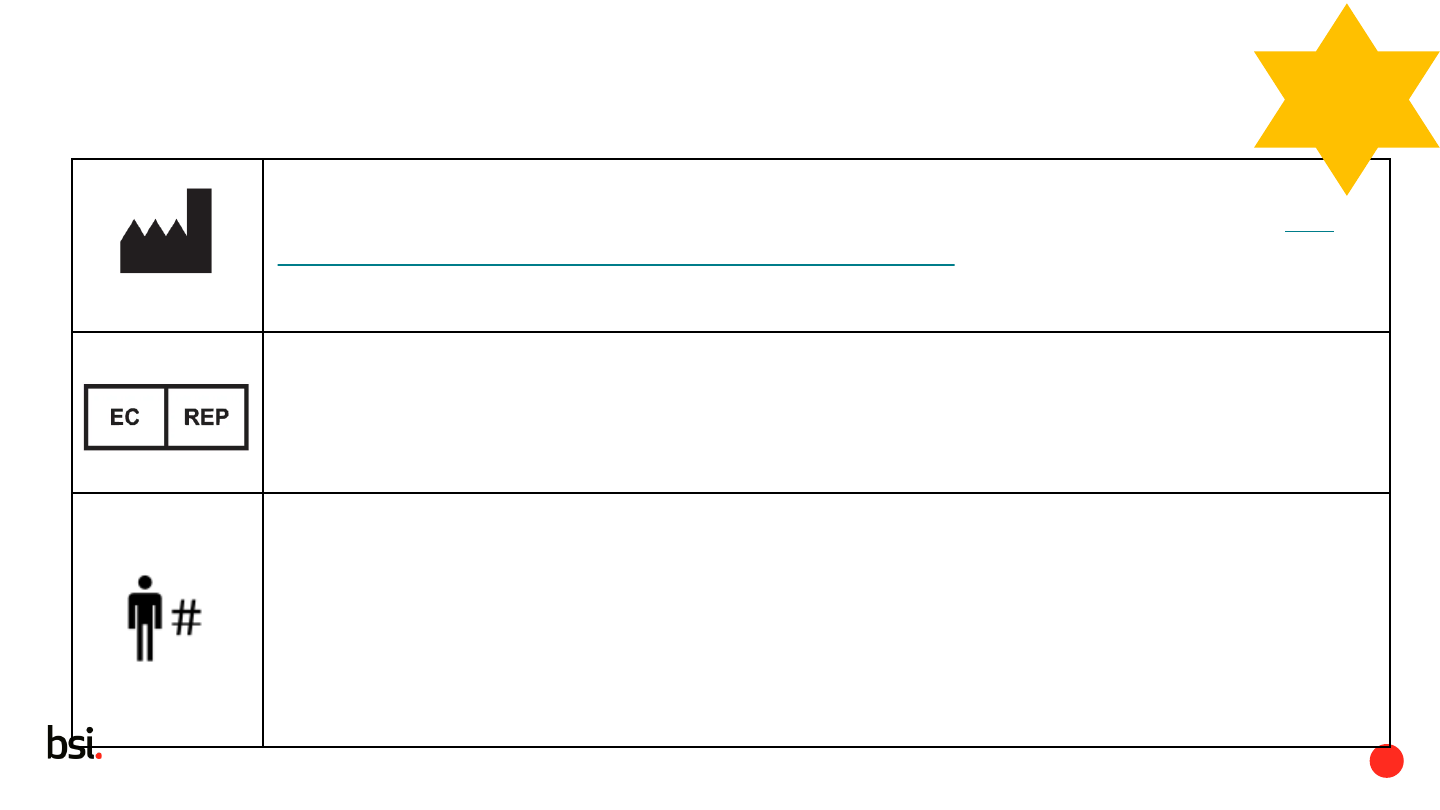
Manufacturer
This symbol shall be accompanied by the name and address of the manufacturer (i.e.
the person placing the medical device on the market),
adjacent to the symbol.
Update to notes; deleted 3 and 6, references to Directives replaced with Regulations
5.1.2
23.2(d)
Authorized representative in the European Community / European Union
Update to notes; If multiple symbols (i.e., Authorized Representative, Importer,
Distributor, Translation, or Repackaging) identify the same responsible entity, the name
and address need not be duplicated.
5.7.1
Article 18
Patient number
Indicates a unique number associated with an individual patient. Added to Notes:
NOTE 1 The hash mark (#) is part of the symbol. The patient number appears adjacent
to the symbol.
NOTE 2 Usage would be to indicate a data entry field or location (e.g. medical device
input screen or implant card) or in information provided to the patient.
Symbols to be used on labelling (ISO 15223)
Revised
Copyright © 2020 BSI. All rights reserved

8
5.2.6
23.2(l)
Do not resterilize
Added restriction:
This symbol is only to be used when there is an accompanying Sterile symbol (5.2.1 to
5.2.5 or 5.2.10).
This symbol is not to be used on reusable medical devices that are intended to be
sterilized between uses.
5.2.8
23.2(l), 23.3(j)
Do not use if package is damaged and consult instructions for use
Updated description; Indicates a medical device that should not be used if the package
has been damaged or opened and that the user should consult the Instructions for Use
for additional information.
Symbols to be used on labelling (ISO 15223)
Revised
Copyright © 2020 BSI. All rights reserved
9
5.4.3
23.2(m)
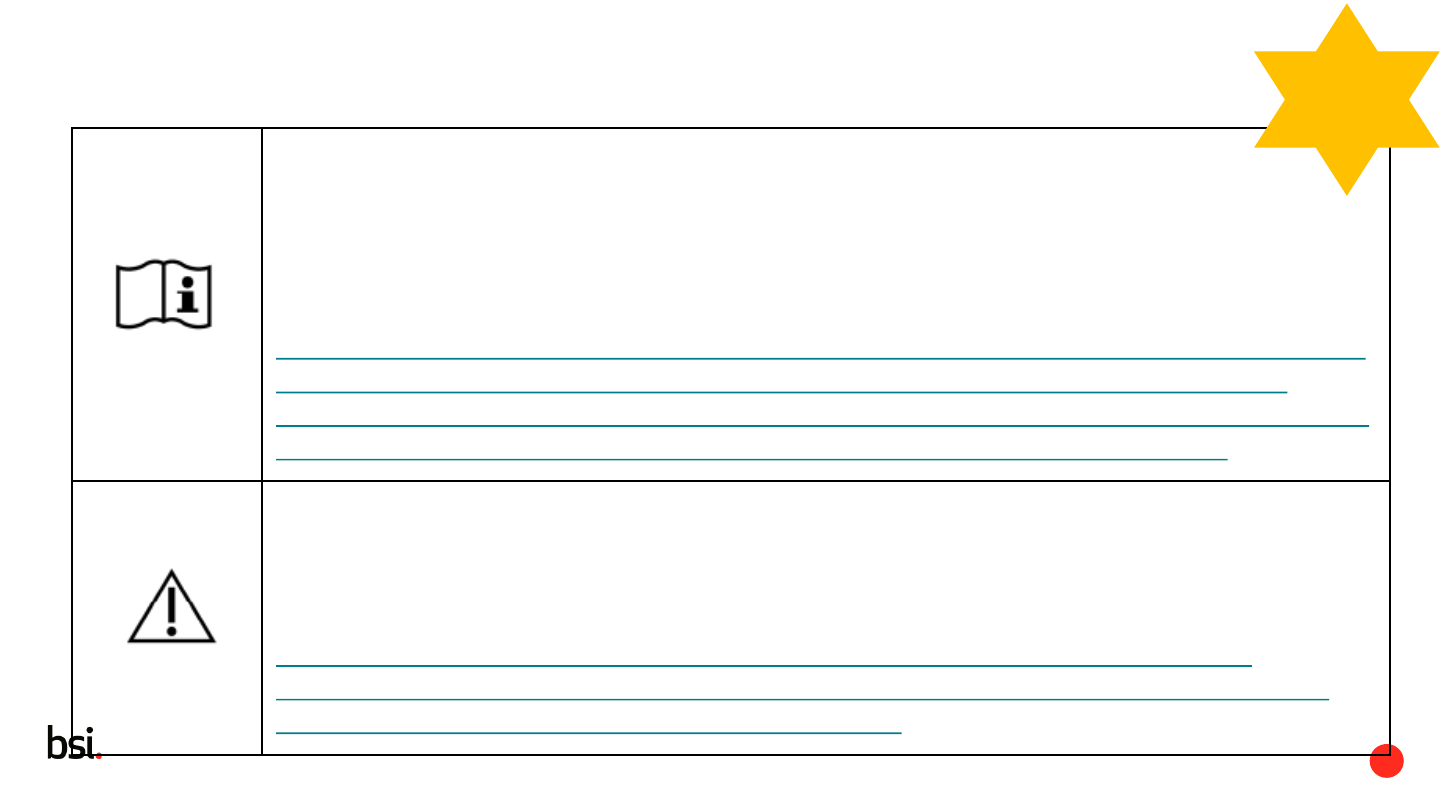
Consult instructions for use or consult electronic instructions for use
Indicates the need for the user to consult the instructions for use
When the instructions for use and patient information are located within the same
electronic instructions for use, a manufacture may choose to use this symbol to indicate
both.
When used to indicate an instruction to consult an electronic instructions for use (eIFU),
this symbol is accompanied by an
eIFU indicator. This indicator may represent the
manufacturer’s
eIFU website or any other appropriate indication on the use of eIFU. The
indicator may be placed either alongside, beneath or surrounding the symbol
5.4.4
23.2(m)
Caution
To indicate that caution is necessary when operating the device or control close to where
the symbol is placed, or to indicate that the current situation needs operator awareness
or operator action in order to avoid undesirable consequences.
Indicates the need for the user to consult the instructions for use for important
cautionary information such as warnings and precautions that cannot, for a variety of
reasons, be presented on the medical device itself.
Symbols to be used on labelling (ISO 15223)
Revised
Copyright © 2020 BSI. All rights reserved

10
5.1.8
Article 13.3
Importer
Indicates the entity importing the medical device into the locale
This symbol shall be accompanied by the name and address of the importing
entity, adjacent to the symbol
5.1.9
Distributor
Indicates the entity distributing the medical device into the locale
This symbol shall be accompanied by the name and address of the importing
entity, adjacent to the symbol
5.1.10
Article 18.1(a)
Annex VI B.11
Model number
To identify the model number or type number of a product
This symbol shall be accompanied by the model number or catalogue number
of the product, adjacent to the symbol.
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved

11
5.1.11
Country of manufacture
To identify the country of manufacture of products
In the application of this symbol, the "CC" shall be replaced by either the two
letter country code or the three letter country code defined in ISO 3166-1.
5.2.10
23.2(l), 23.3(c)
Sterilized using vaporized hydrogen peroxide
Indicates a medical device that has been sterilized using vaporized hydrogen
peroxide
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved

12
5.2.11
23.3(a)
Single sterile barrier system
Indicates a single sterile barrier system
This symbol shall be placed adjacent to or in combination with symbol 5.2.1, 5.2.2,
5.2.3, 5.2.4, 5.2.5, 5.2.9 or 5.2.10
NOTE 1 A solid line identifies a sterile barrier system.
NOTE 2 Additional information can found in ISO 11607-1 and ISO 11607-2.
5.2.12
23.3(a)
Double sterile barrier system
Indicates two sterile barrier systems
This symbol shall be placed adjacent to or in combination with symbol 5.2.1, 5.2.2,
5.2.3, 5.2.4, 5.2.5, 5.2.9 or 5.2.10
NOTE 1 A solid line identifies a sterile barrier system.
NOTE 2 Additional information can found in ISO 11607-1 and ISO 11607-2.
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved

13
5.2.13
23.3(a)
Single sterile barrier system with protective packaging inside
Indicates a single sterile barrier system with protective packaging inside
This symbol shall be placed adjacent to or in combination with symbol 5.2.1, 5.2.2, 5.2.3, 5.2.4, 5.2.5,
5.2.9 or 5.2.10
NOTE 1 The protective packaging located inside the sterile barrier system is designed to prevent
damage to the contents or to help with aseptic presentation. It does not provide a microbial barrier to
maintain sterility.
NOTE 2 Additional information can found in ISO 11607-1 and ISO 11607-2.
5.2.14
23.3(a)
Single sterile barrier system with protective packaging outside
Indicates a single sterile barrier system with protective packaging outside
This symbol shall be placed adjacent to or in combination with symbol 5.2.1, 5.2.2, 5.2.3, 5.2.4, 5.2.5,
5.2.9 or 5.2.10
NOTE 1 The protective packaging located outside the sterile barrier system is designed to prevent
damage to the sterile barrier system and the contents. The protection can be against physical hazards,
particulate contamination or other environmental hazards, but it does not include a microbial barrier.
NOTE 2 Additional information can found in ISO 11607-1 and ISO 11607-2.
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved
14
5.4.6
23.2(e)
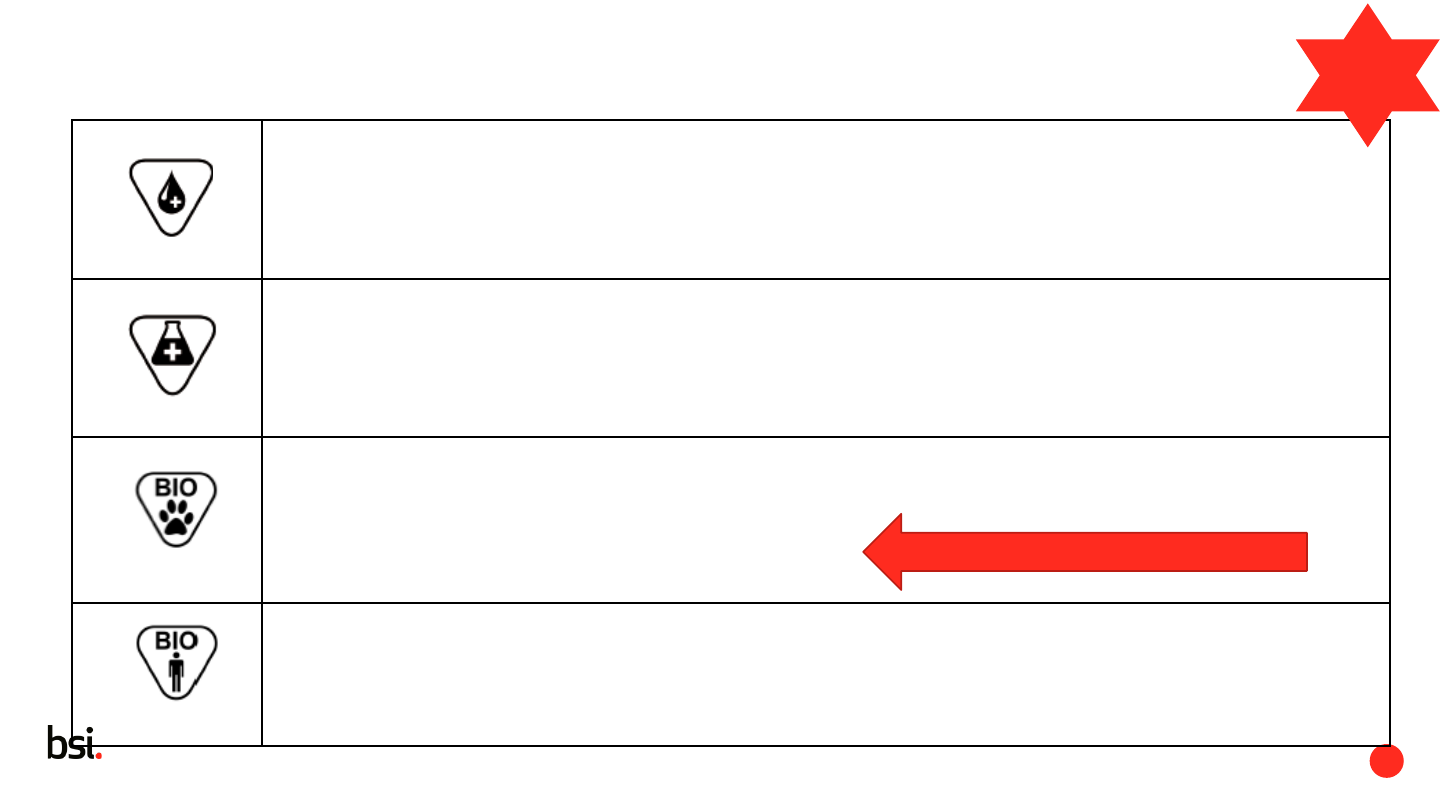
Contains human blood or plasma derivatives
Indicates a medical device that contains or incorporates human blood or plasma derivatives.
The embedded cross may be deleted or replaced with another element appropriate with cultural
requirements
5.4.7
23.2(e)
Contains a medicinal substance
Indicates a medical device that contains or incorporates a medicinal substance
The embedded cross may be deleted or replaced with another element appropriate with cultural
requirements
5.4.8
23.2(e)
Contains biological material of animal origin
Indicates a medical device that contains biological tissue, cells, or their derivatives, of animal origin
GSPR: as referred to in Regulation (EU) No 722/2012
bovine, ovine, caprine, deer, elk, mink, cats
5.4.9
23.2(e)
Contains biological material of human origin
Indicates a medical device that contains biological tissue, cells, or their derivatives, of human origin
Symbols to be used on labelling (ISO 15223)
New
Non-TSE susceptible species?
Copyright © 2020 BSI. All rights reserved

15
5.4.10
10.4.5
Contains hazardous substances
Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic
(CMR), or substances with endocrine disrupting properties
5.4.11
Contains nano materials
Indicates a medical device that contains nano materials
5.4.12
ISO 20417 3.26
Single patient - multiple use
Indicates a medical device that may be used multiple times (multiple procedures) on a single patient
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved

16
5.7.2
Article 18
Patient name
Indicates the name of the patient
NOTE Usage would be to indicate a data entry field or location (e.g. medical device input screen or
implant card) or in information provided to the patient.
5.7.3
Article 18
Patient identification
Indicates the identification data of the patient
NOTE Usage would be to indicate a data entry field or location (e.g. medical device input screen or
implant card) or in information provided to the patient
5.7.4
Article 18
Patient information website
Indicates a website where a patient may obtain additional information on the medical product
This symbol shall be accompanied by the website information (url) adjacent to the symbol
NOTE Usage would be to indicate a data entry field or location on an implant card or in information
provided to the patient
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved

17
5.7.5
Article 18
Health care centre or doctor
To indicate the address of the health care centre or doctor where medical information about the patient
may be found
This symbol shall be accompanied, adjacent to the symbol, by the address of the health care centre or
doctor
NOTE 1 The embedded cross can be deleted or replaced with another element appropriate with
cultural requirements.
NOTE 2 Usage would be to indicate a data entry field or location (e.g. medical device input screen or
implant card) or in information provided to the patient
5.7.6
Article 18
Date
To identify the date that information was entered or a medical procedure took place
This symbol shall be accompanied, adjacent to the symbol, by the date appropriate for the use of the
symbol
NOTE Usage would be to indicate a data entry field or location (e.g. medical device input screen or
implant card) or in information provided to the patient
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved
18
5.7.7
23.2(q)
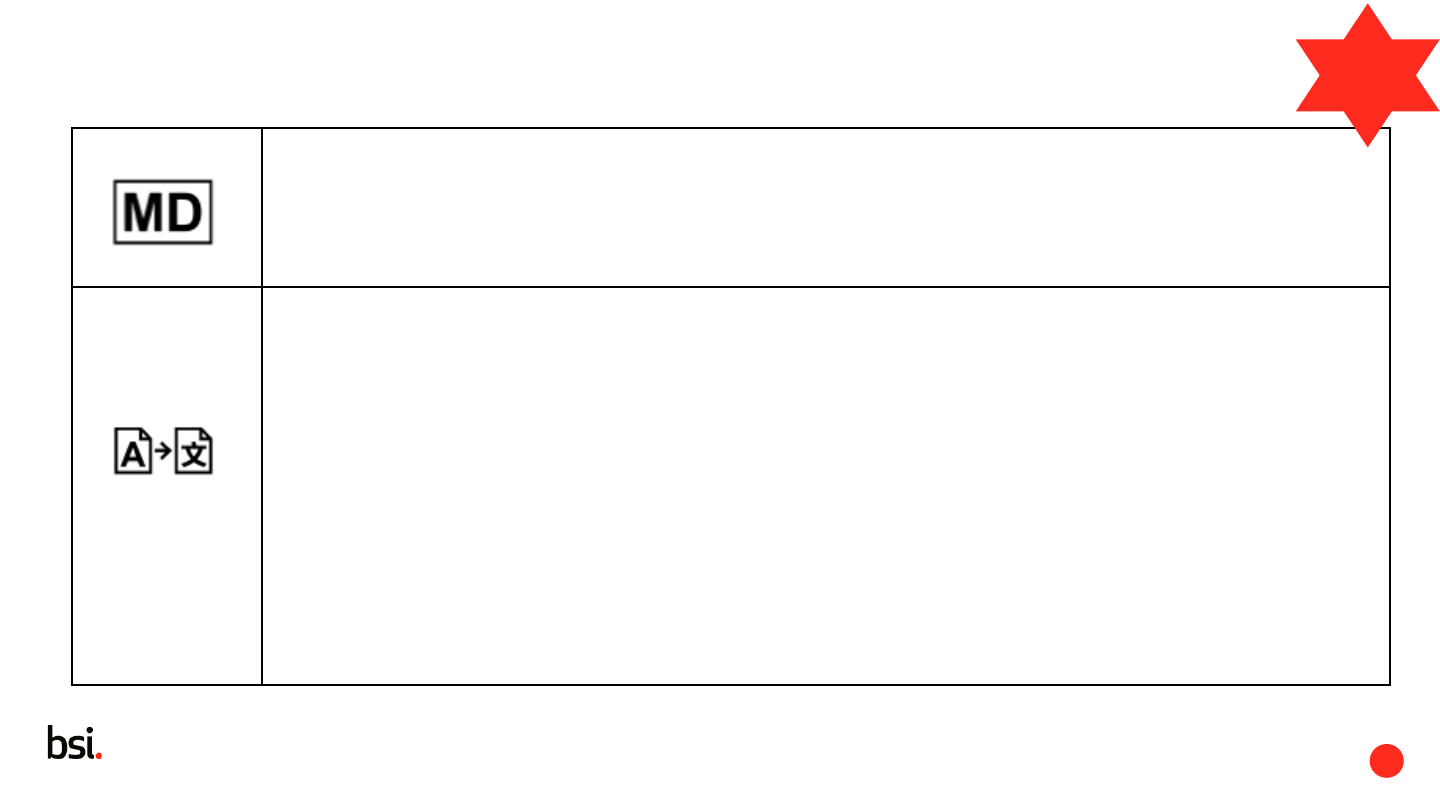
Medical device
Indicates the item is a medical device
5.7.8
Article 16.3
Translation
To identify that the original medical device information has undergone a translation which supplements
or replaces the original information
This symbol shall be accompanied by the name and address of the entity that is responsible for the
translation activity, adjacent to the symbol.
NOTE 1 Translation activities cannot be allowed in all jurisdictions, however for some locales,
translation activities are required to meet local language requirements.
NOTE 2 If multiple symbols (i.e., Authorized Representative, Importer, Distributor, Translation, or
Repackaging) identify the same responsible entity, the name and address need not be duplicated.
This symbol should only be used when the translation activity was undertaken by someone other than
the manufacturer.
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved

19
5.7.9
Article 16.3
Repackaging
To identify that a modification to the original medical device packaging configuration has occurred
This symbol shall be accompanied by the name and address of the entity that is responsible for the
repackaging activity, adjacent to the symbol
NOTE 1 Repackaging activities cannot be allowed in all jurisdictions, however for some locales, a
change in the outer packaging, including changes to the pack size is needed or required.
NOTE 2 Depending on the jurisdiction, additional information (i.e. date of repackaging) can be needed.
NOTE 3 If multiple symbols (i.e., Authorized Representative, Importer, Distributor, Translation, or
Repackaging) identify the same responsible entity, the name and address need not be duplicated.
This symbol should only be used when the repackaging activity was undertaken by someone other
than the manufacturer.
5.7.10
23.2(h)
Unique Device Identifier
Indicates a carrier that contains Unique Device Identifier information
The use of this symbol is optional, but may be used when multiple data carriers are present on the
label. If used, this symbol shall be placed adjacent to the Unique Device Identifier carrier.
NOTE Used to identify which information is associated with Unique Device Identifier
Symbols to be used on labelling (ISO 15223)
New
Copyright © 2020 BSI. All rights reserved
20
No change Update to notes, Restrictions,
Additional requirements
5.2.2
5.2.7
5.3.1
5.3.4
5.3.5
5.3.6
5.3.7
5.4.1
5.4.2
5.4.5
5.5.3
5.5.4
5.5.6
5.1.3
5.1.4
5.1.5
5.1.6
5.1.7
5.2.1
5.2.3
5.2.4
5.2.5
5.2.6
5.2.8
5.2.9
5.3.2
5.3.3
5.3.8
5.3.9
5.5.1
5.5.2
5.5.5
5.6.1
5.6.2
5.6.3
5.6.4
5.6.5
5.6.6
Symbols to be used on labelling (ISO 15223)
Copyright © 2020 BSI. All rights reserved

21
Symbols to be used on labelling (ISO 15223)
A. Annex A – examples
B. Annex B – Use of general prohibition symbol and negation symbol - unchanged
C. Annex C – Terminology - Alphabetized index of defined terms
Copyright © 2020 BSI. All rights reserved
22
Symbols to be used on labelling (ISO 15223)
MDD/AIMD/IVDD
• ISO 15223:2020 would represent the state of the art for the Medical Device Directives.
• Conduct gap analysis and risk assessment.
• Should have a plan to implement the 2020 revision (once available) for BSI review.
MDR/IVDR
• ISO 15223:2020 would represent the state of the art for the Medical Device Directives and Regulation.
• It is anticipated the 2020 revision will be harmonized to the Regulations at the point of publication.
• Conduct risk assessment.
In areas for which no harmonised standards or CS exist,
the symbols and colours shall be described in the
documentation supplied with the device.
GSPR 23.1(h) / 20.1(h)
Copyright © 2020 BSI. All rights reserved
23
Symbols to be used on labelling (ISO 15223)
Current Status
• Final draft international standard is being prepared
• Commenting ongoing for the harmonisation
• Publication remains anticipated in 2020
Copyright © 2020 BSI. All rights reserved

24
Summary
• ISO 15223:2020 expected in Q4 2020
• General updates to definitions & descriptions
• New symbols to meet regulatory requirements
• MDD gap analysis
• Define symbols for MDR
Copyright © 2020 BSI. All rights reserved

25
Information to be supplied by the manufacturer (ISO 20417)
New standard replaces EN 1041:2008
General requirements for identification
and labels on:
medical device or accessory
packaging
marking of a medical device or
accessory
accompanying information
Does not specify the means by which
the information is to be supplied
Terms and definitions aligned to:
ISO 7010:2019
ISO 13485:2016
ISO 14971:2019
ISO 15223-1:—
ISO 16142-1:2016
ISO 16142-2:2017
IEC 62366-1:2015+AMD1:2019
Copyright © 2020 BSI. All rights reserved

26
Information to be supplied by the manufacturer (ISO 20417)
ISO 20417:2020
• Main body of standard: 7 clauses, 35 pages
• 9 Annexes (informative): 37 pages
EN 1041:2008
• Main body of standard: 6 clauses, 5 pages
• 4 Annexes (informative): 14 pages
Copyright © 2020 BSI. All rights reserved

27
Information to be supplied by the manufacturer (ISO 20417)
1 Scope
2 Normative references
3 Terms and definitions
4 General considerations
5 Information elements to be established
6 Requirements for accompanying information
7 Other information which is required to be supplied with the medical device or accessory
CONTENTS
Copyright © 2020 BSI. All rights reserved

28
Annex A (informative) Particular guidance and rationale
Annex B (informative) Example test method for assessing clearly legible
Annex C (informative) Example test method for assessing durability
Annex D (informative) Cross reference between the document and the requirements considered
Annex E (informative) Reference to the IMDRF essential principles and labelling guidances
Table E.1 — Correspondence between this document and the essential principles
Table E.2 — Correspondence between this document and the labelling principles
Annex F (informative) Reference to the essential principles
Table F.1 — Correspondence between the essential principles for non-IVD medical devices and this document
Table F.2 — Correspondence between the essential principles for IVD medical devices and this document
Annex G (informative) Reference to the general safety and performance requirements for medical devices
Annex H (informative) Reference to the general safety and performance requirements for IVD medical devices
Annex I (informative) Terminology — Alphabetized index of defined terms
Bibliography
Information to be supplied by the manufacturer (ISO 20417)
CONTENTS
Copyright © 2020 BSI. All rights reserved

29
Information to be supplied by the manufacturer (ISO 20417)
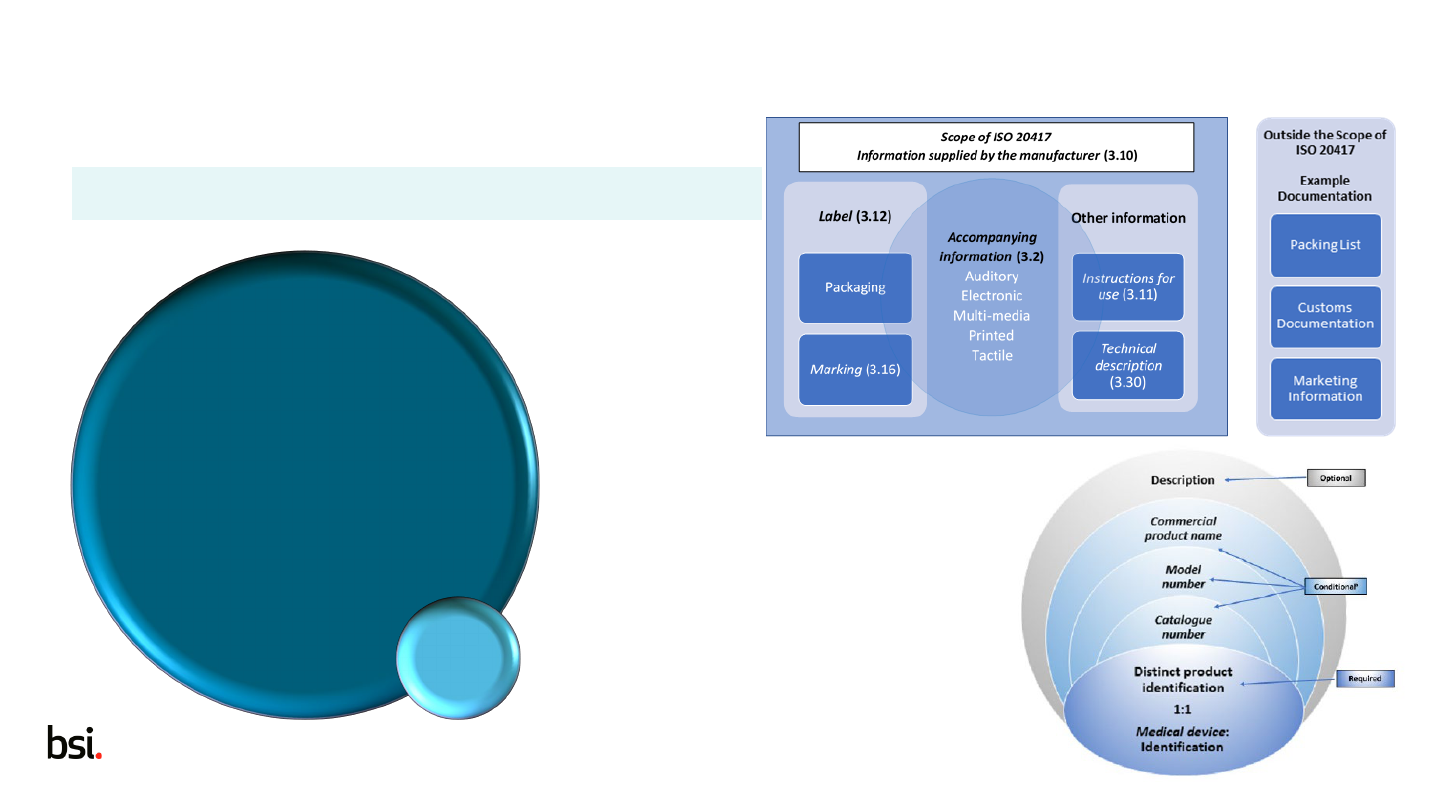
1 Scope
ISO 20417:2020
• Information supplied by the manufacturer for a medical device or accessory
• Includes MD, AIMD, IVD
EN 1041:2008
• Information supplied by the manufacturer for a medical device or accessory
• Includes MD, AIMD
Copyright © 2020 BSI. All rights reserved
30
Information to be supplied by the manufacturer (ISO 20417)
2 Normative references
ISO 20417:2020
• Describes standards and other documents required for the application of ISO 20417
• Expanded / updated list vs 1041 (ISO 3166-1, ISO 639-1, ISO 1000, ISO 8601)
ISO 639-1:2002, Codes for the representation of names of languages – Part 1: Alpha-2 Code
ISO 639-2:1998, Codes for the representation of names of languages – Part 2: Alpha-3 code
ISO 639-3:2007, Codes for the representation of names of languages – Part 3: Alpha-3 code for comprehensive 164 coverage of languages
ISO 3166-1:2013, Codes for the representation of names of countries and their subdivisions – Part 1: Country codes
ISO 3864-1:2011, Graphical symbols - Safety colours and safety signs - Part 1: Design principles for safety signs 167 and safety markings
ISO 7000 (database), Graphical symbols for use on equipment – Registered symbols
ISO 7010:2019, Graphical symbols – Safety colours and safety signs – Registered safety signs
ISO 8601-1:2019, Date and time — Representations for information interchange — Part 1: Basic rules
ISO 13485:2016, Medical devices – Quality management systems – Requirements for regulatory purposes
ISO 14971:2019, Medical devices – Application of risk management to medical devices
ISO 15223-1:—2, Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements
ISO 16142-1:2016, Medical devices – Recognized essential principles of safety and performance of medical devices – Part 1: General essential principles and additional
specific essential principles for all non-IVD medical devices and guidance on the selection of standards
ISO 16142-2:2017, Medical devices – Recognized essential principles of safety and performance of medical devices – Part 2: General essential principles and additional
specific essential principles for all IVD medical devices and guidance on the selection of standards
IEC 60417 (database), Graphical symbols for use on equipment
IEC 62366-1:2015+AMD1:2019, Medical devices – Part 1: Application of the usability engineering process to medical devices
ISO 80000-1:2009, Quantities and units – Part 1: General
Copyright © 2020 BSI. All rights reserved
31
Information to be supplied by the manufacturer (ISO 20417)
3 Terms and definitions
20417
(31)
1041
(6)
Definitions aligned to
expanded normative
references
Reflect the increase in detail
of the new standard
Copyright © 2020 BSI. All rights reserved

32
Information to be supplied by the manufacturer (ISO 20417)
5 Information elements to be established
ISO 20417:2020 EN 1041
4 The risk management process of ISO 14971:2019 and the usability engineering process of IEC
62366-1:2015+AMD1:2019 should be used to determine the information, including information
for safety, to be provided in the information supplied by the manufacturer
4.1
4 Language, special skills, use environment -
4 Requirement to be understandable by the intended user 5.2.2
Copyright © 2020 BSI. All rights reserved

33
Information to be supplied by the manufacturer (ISO 20417)
5 Information elements to be established
ISO 20417:2020 EN 1041
5.1 Units of measurement ISO 80000-1:2009 4.2
5.2 Graphical information
- Explain in accompanying information – unless not required
- Symbols standards referenced
4.2
5.3 - Clearly identify the language. Refers to ISO 639
- Clearly identify country if multiple contact details. Refers to ISO 3166-1:2013
4.3
5.4 Unless specified by jurisdiction use date format as ISO 8601-1:2019 4.4
5.5 - Specifies address – street/road, number/house/floor, city, state/region, postal code, country
- street/road and number/house/floor may be omitted if a postal code covers this
- Prohibits PO Box
5.1.2
Copyright © 2020 BSI. All rights reserved

34
Information to be supplied by the manufacturer (ISO 20417)
5 Information elements to be established
ISO 20417:2020 EN 1041
5.6 Commercial product name -
5.7 Model number
- medical device, an accessory or a medical device family that have shared characteristics
- may be associated with multiple catalogue numbers
-
5.8 Catalogue number
- assigned to medical device, or a combination of medical devices or accessories
- unique catalogue number shall be related to a single, defined product specification
- Multiple catalogue numbers may be associated with a single model number
-
5.9 Production controls – use at least one of:
- lot number
- serial number
- for medical devices containing cell tissues, donor identification information
- year and month by which it is to safe to use
- year and month of manufacture
4.5.3
Copyright © 2020 BSI. All rights reserved

35
Information to be supplied by the manufacturer (ISO 20417)
5 Information elements to be established
ISO 20417:2020 EN 1041
5.10 Unique device identifier - If required by the authority having jurisdiction
This identifier shall have a 1:1 relation to:
- a single catalogue number
- a single model number
- a single commercial product name
-
5.11 Types of use/reuse assigned at the level of model number or catalogue number
- single use
- single patient multiple use
- multiple patient multiple use
-
5.12 Sterile – identify as sterile and with method
Sterile/non-sterile versions should have different model or catalogue numbers
-
Copyright © 2020 BSI. All rights reserved
36
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.1 Requirements for information to be supplied on the label -
6.1.1 Minimum requirements for the label
Human readable
Marked on device where possible
Unless omission of these markings does not adversely affect the benefit/risk balance
-
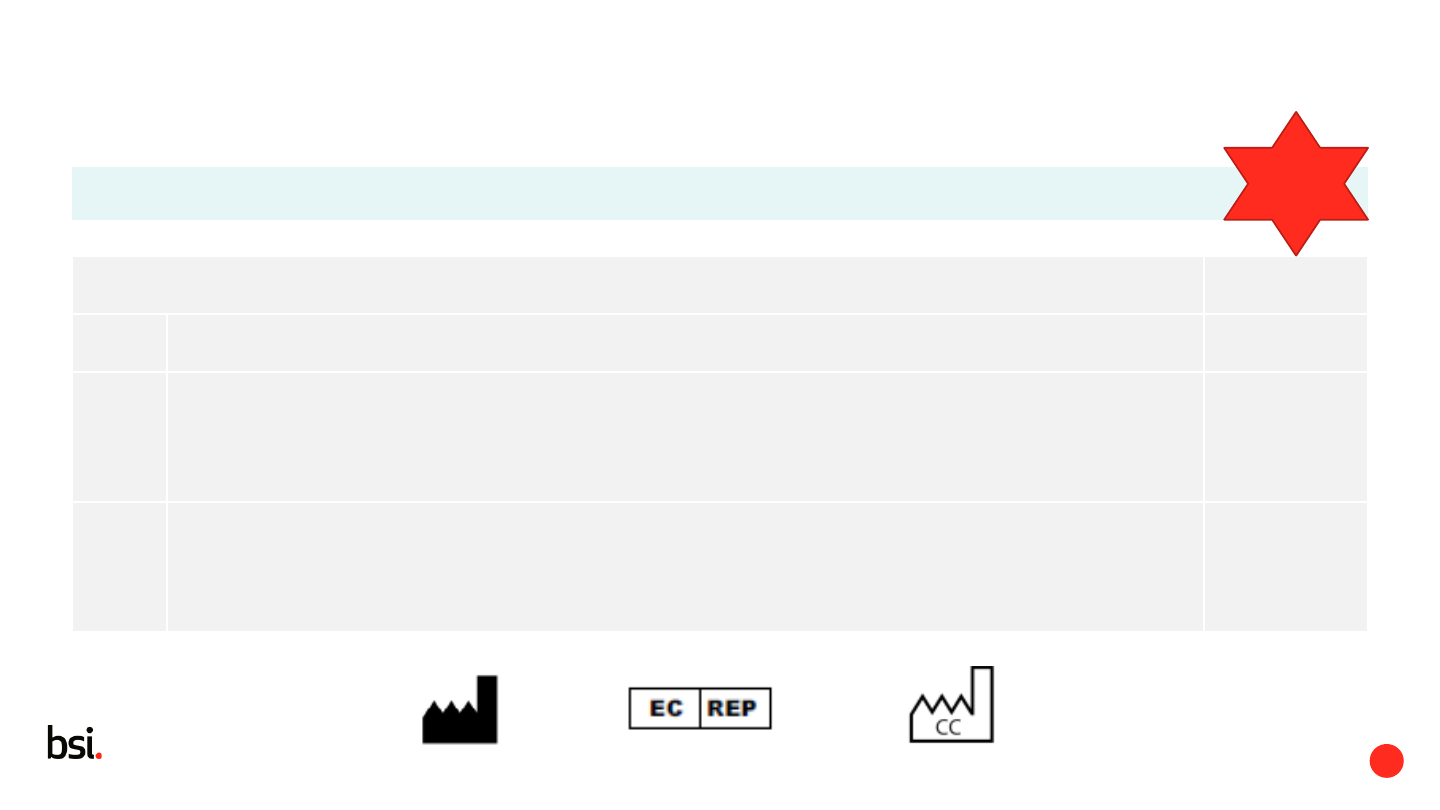
6.1.2 Label of a medical device or accessory shall include
- Name or trade name and the full address of the manufacturer and
- Authorised representative
- Country of manufacture may be used (refers to standards)
-
label
Copyright © 2020 BSI. All rights reserved
37
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
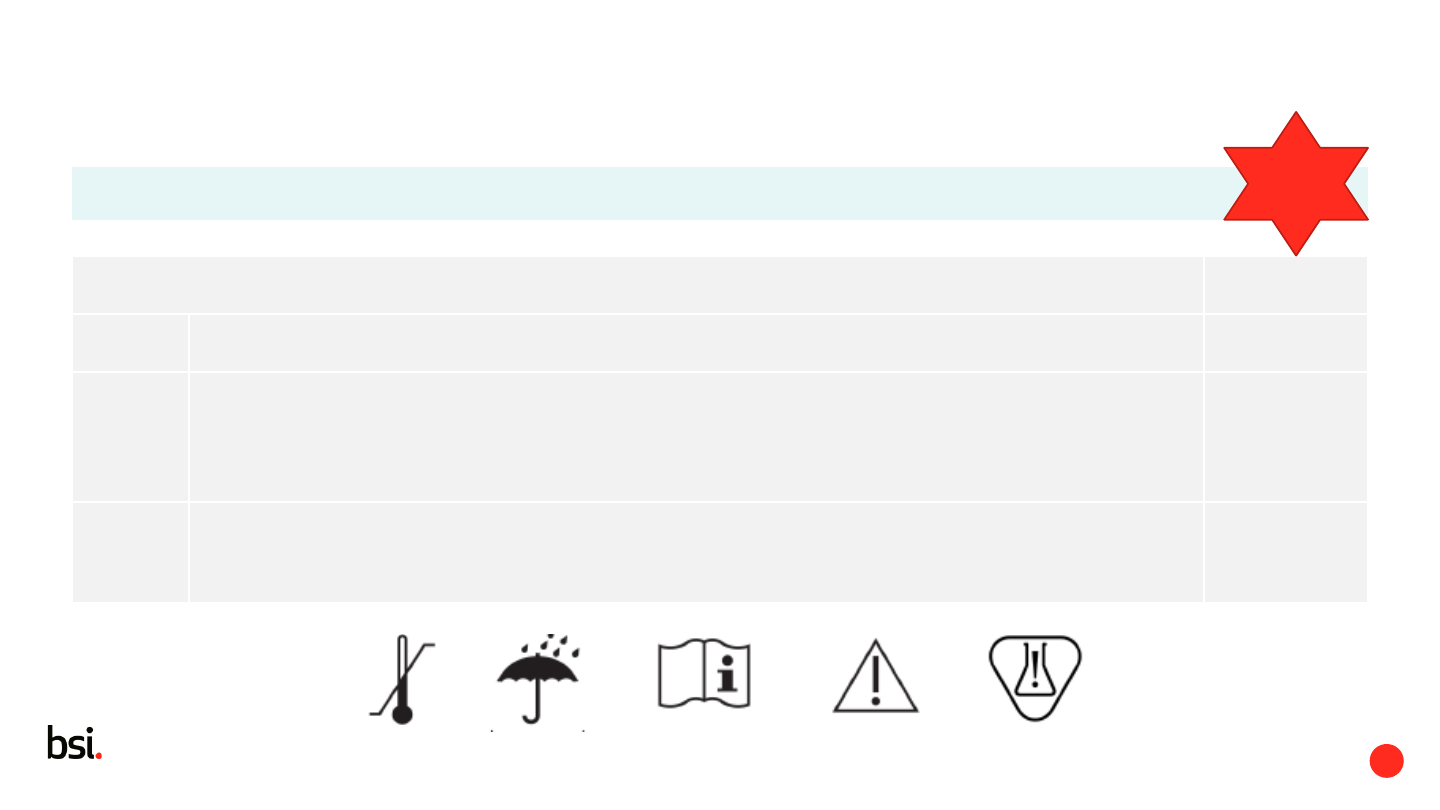
6.1.3 a Identify the device and its use on the label. Model/catalogue/name/description -
6.1.3 b - Any special storage conditions
- Any special operating instructions for immediate attention of the user
- Any warnings or precautions for immediate attention of the user
May be kept to a minimum, in which case, more detailed information in the IFU
-
6.1.3 c Contains known allergens as well as phthalates or other substances
In a concentration that is above 0,1 % weight by weight
That are classified as endocrine disrupting, carcinogenic, mutagenic or toxic to reproduction
-
label
Copyright © 2020 BSI. All rights reserved
38
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
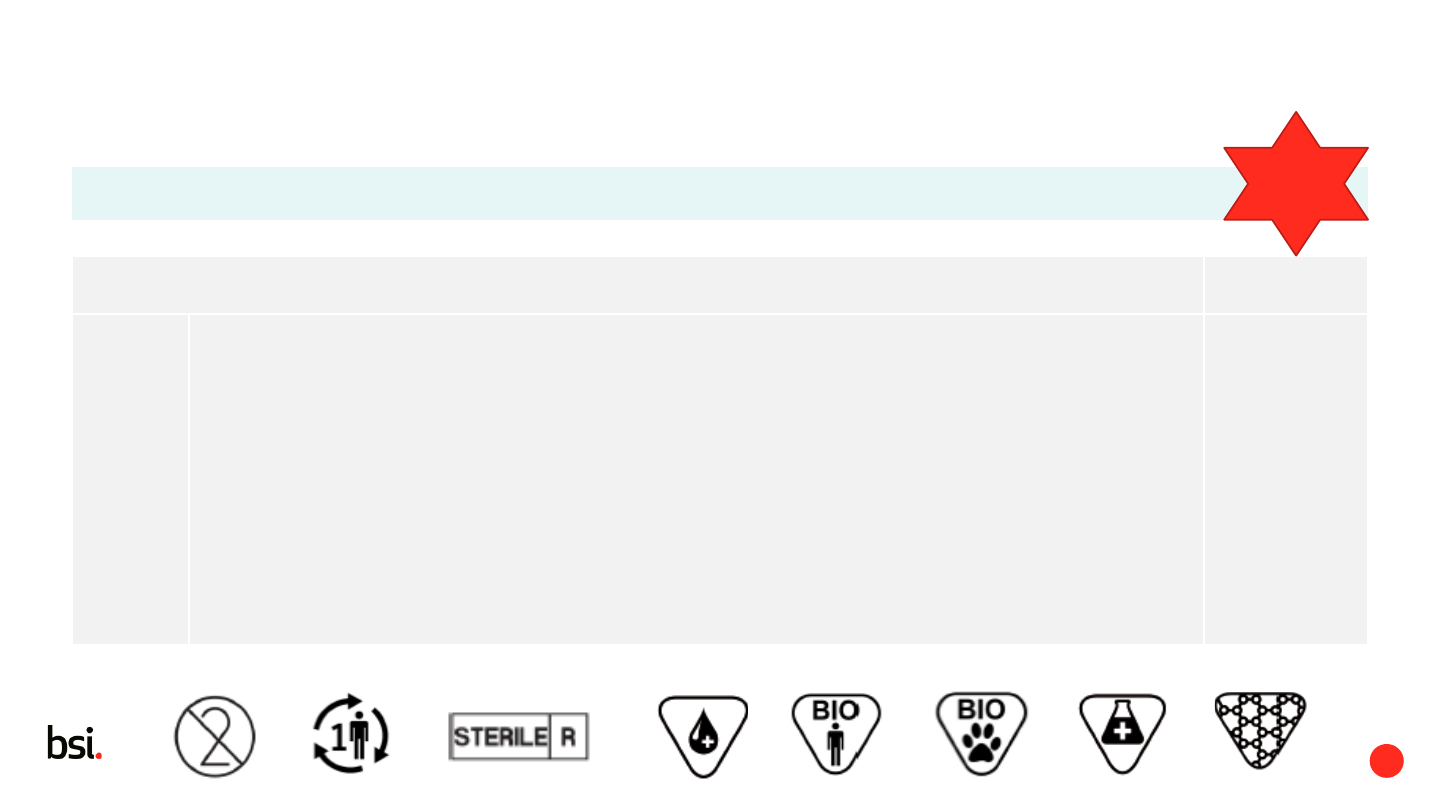
6.1.3 d Label must include (if applicable):
eIFU
Single use / do not re-use / single use only
Single patient multiple use
Limitations on re-use
Sterile and method or symbol
human blood or plasma derivatives
biological material of human origin
biological material of animal origin
medicinal substances
nanotechnology materials
-
label
Copyright © 2020 BSI. All rights reserved
39
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
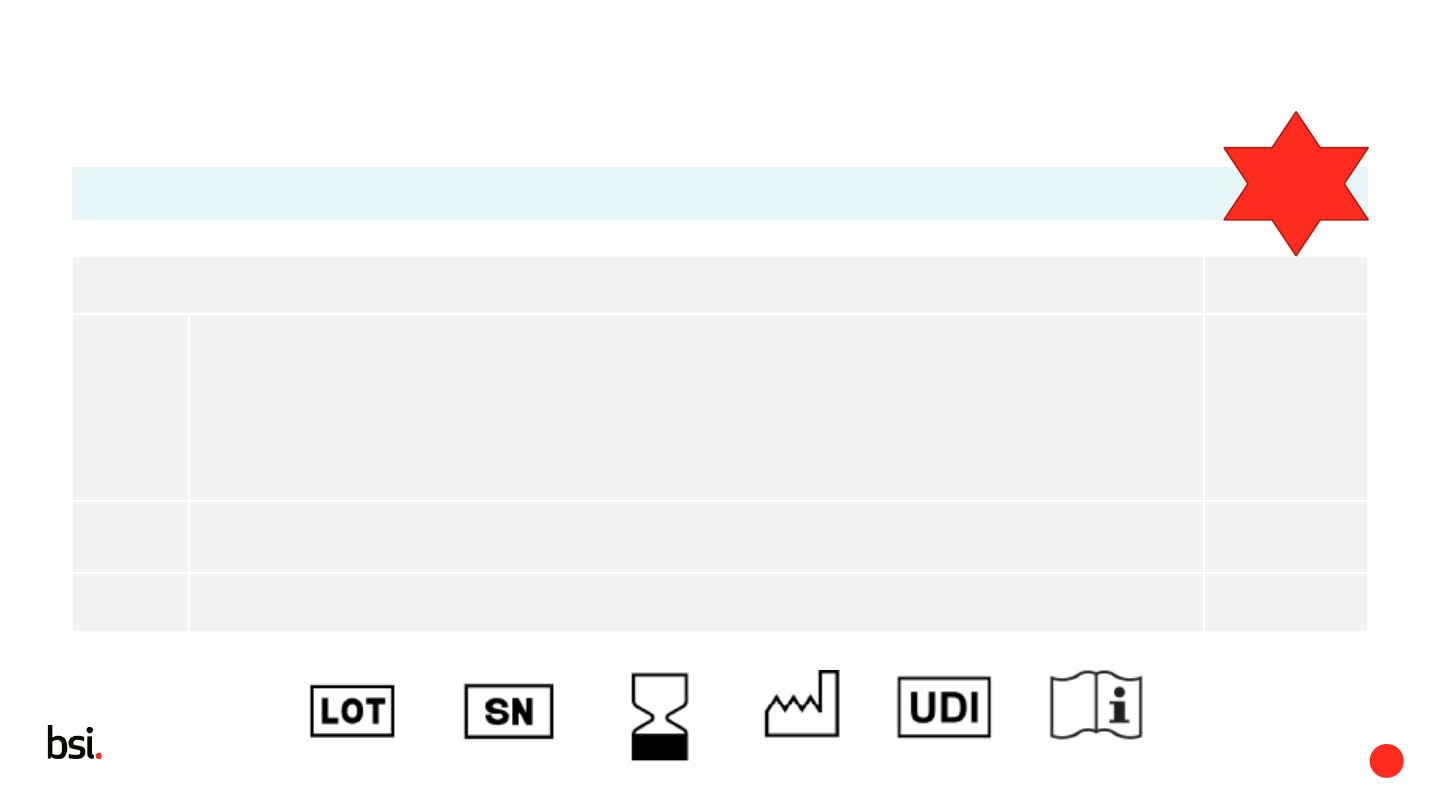
6.1.4 Label must include (if applicable):
Lot number
Serial number
Expiry date
Date of manufacture (if no expiry date)
UDI
-
6.1.5 Label must include (if applicable):
Consult IFU
-
6.1.6 Safety sign (if needed) as per ISO 7010 -
label
Copyright © 2020 BSI. All rights reserved

40
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.2 Identification requirements for detachable components of a medical device or accessory
Use model, name, symbols etc
-
6.3 Legibility of the label
- Legible when viewed from the intended position of user performing the related function
- See Annex B
5.2.3
6.4 Durability of markings
- Sufficiently durable to remain clearly legible over expected lifetime or shelf life
- Clearly legible under reasonably foreseeable environmental and mechanical impacts of use
- Removable only with an object that can be used to secure or release a fastener or by force
- See Annex C
5.2.4
label
Copyright © 2020 BSI. All rights reserved
41
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.5.1 Information to be provided on the packaging:
name or trade name and address
authorized representative
unique device identifier
Lot number
Serial number
For medical devices containing cell tissues, donor identification information
Expiry date
Manufacture date
Distinctive identification (model, catalogue number)
Single patient / multiple use
-
Pack
Copyright © 2020 BSI. All rights reserved
42
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.5.2 Packaging for the lay user:
In addition to the requirements of 6.5.1, if the medical device or accessory is intended to be
presented to the lay user for retail sales and is intended for use by a lay user, the sales
packaging shall allow the identification of the information needed by the lay user including, as
a minimum all of the following:
- statement of intended use, unless the purpose is obvious to the intended lay user
- the information needed to select the proper size, if applicable
- any special requirements for a battery-powered
- any necessary contraindications, warnings or precautions for immediate attention of the user
- safe disposal information
- visible under expected conditions of sale
-
Pack
Copyright © 2020 BSI. All rights reserved

43
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.5.3 Special conditions indicated on the packaging:
- Special handling measures to be taken during transport or storage
- Premature unpackaging safety signs
- Sterile / method
- Expiry date
- Indication permitting the sterile packaging to be recognized as such
- What to do if the sterile packaging is damaged or unintentionally opened before use
-
Pack
Copyright © 2020 BSI. All rights reserved
44
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6 Requirements for information in the instructions for use and technical description -
6.6.1 information accompanying the medical device or accessory shall include:
- the information specified for inclusion in the instructions for use
- the information specified for inclusion in the technical description
- the justification for any omission shall be evaluated according to ISO 14971:2019.
the information specified for inclusion in the technical description may be included in the IFU
-
IFU
portion of the accompanying information directed to the responsible organization
and service personnel that is essential for preparation for the first use and safe use,
maintenance or repair as well as processing, transport or storage for the expected
lifetime of a medical device
Note 1 to entry: The technical description may be included in the instructions for use
Copyright © 2020 BSI. All rights reserved

45
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6 Requirements for information in the instructions for use and technical description -
6.6.1 name or trade name and full address of manufacturer and authorised representative
- Contact information of the manufacturer
- Device identity (commercial product name / device family name / model / catalogue number)
- Device description
-
6.6.1 may include separate accompanying information for the professional user and the lay user
- Consistent
- State the version
- Readily understandable
- Supplemented by drawings / diagrams
-
IFU
Copyright © 2020 BSI. All rights reserved
46
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 The instructions for use shall document all the following
- the general information of 6.6.1
- the use of the medical device or accessory as intended by the manufacturer
- information for safety necessary to safely use the device in accordance with specifications
- the ‘statement of intended use’
- claimed performance
- explanation of any residual risk, including any foreseeable adverse events or side effects
- for the user or conveyed by user to patient
-
6.6.2 Residual risk conveyed as:
- limitations
- contraindications
- precautions
- warnings
-
IFU
Copyright © 2020 BSI. All rights reserved
47
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 Unique identifier for IFU -
6.6.2 Parts of the medical device that cannot be safely serviced or maintained while in use with patient -
6.6.2 Safe disposal consider infection / microbial / environmental / physical hazards -
6.6.2 Preparatory treatment or handling of the device before ready for use -
6.6.2 The information contained in 6.1.2, 6.1.3 and 6.5.3 -
IFU
The instructions for use should contain only the information
most likely to be useful to the user or responsible organization
Copyright © 2020 BSI. All rights reserved
48
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 Include specifications the user requires to use the medical device appropriately -
6.6.2 Information to determine whether the device is ready to perform safely and as intended
- Final assembly / calibration
- Acceptance or performance testing
- Acceptance criteria
-
6.6.2 Nature, and frequency, of preventative and regular maintenance -
6.6.2 Identification of any consumable components and how to replace them -
6.6.2 Calibration and control of risk associated with installation, calibration, servicing -
IFU
Copyright © 2020 BSI. All rights reserved
49
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 Quality control procedures to verify that the device performs as intended
- Procedure
- Instructions on frequency of use
- Limitations of procedure
- How the user should interpret procedure results, including description of acceptance
- Actions to take for test failure
-
6.6.2 Requirements for
- Sterile field
- Training
- User qualifications
-
IFU
Copyright © 2020 BSI. All rights reserved

50
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 If device supplied sterile, include instructions to be followed in the event of the sterile packaging
being damaged or unintentionally opened before use
-
6.6.2 If device supplied non-sterile with the intention that it is sterilized before use, include the
appropriate processing instructions for sterilization
-
6.6.2 Reusable devices:
- processing information
- when it can no longer be used or maximum reuses
-
IFU
Copyright © 2020 BSI. All rights reserved

51
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 Include information of any warnings, precautions, measures to be taken and limitations of use -
6.6.2 User and patient to report any serious incident -
6.6.2 Where multiple devices are provided to single user / location, may provide single copy of IFU -
6.6.2 Identify medicinal or biological substance - quantity, proportion, strength if in direct contact -
6.6.2 Applicable instructions if packaging damaged, unintentionally opened, excursions in storage -
6.6.2 Text on electronic displays intended for the user, shall be clearly legible -
6.6.2 Limitations or incompatibilities in the choice of substances to be delivered -
IFU
Copyright © 2020 BSI. All rights reserved

52
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.2 - Nature / type / intensity / distribution / recommended dose of the emitted radiation
- Ways of avoiding misuse and of appropriately reducing the risks
- Means of protecting the patient, the user, or a third party from unintended radiation
-
6.6.3 Additional requirements for the instructions for use for a lay user -
6.6.3 Identify the patient is an intended user or that the medical device is intended for self-testing -
6.6.3 Circumstances when the user should consult with a healthcare professional -
6.6.3 Warning against servicing and maintenance while in use -
6.6.3 Functions safe / not safe to use / maintenance -
IFU
Copyright © 2020 BSI. All rights reserved

53
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.3 Format appropriate for lay user -
6.6.3 Enable lay user to understand results / confirm it is operating as intended -
6.6.3 Identify the patient is an intended user or that the medical device is intended for self-testing -
6.6.4 Requirements for technical description -
6.6.4 If separate from IFU include 6.6.1 and 6.6.2 a -
6.6.4 If separate from IFU include testing / maintenance / characteristics / accuracy / precision -
6.6.4 If separate from IFU include unique reference -
IFU
Copyright © 2020 BSI. All rights reserved
54
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.4 Data that is essential for safe use, transport and storage, maintenance or repair, and measures or
conditions necessary for installing the medical device and preparing it for use
-
6.6.4 Purpose of the electronic interface / software / intended user of interface -
6.6.4 Communication format, interface specifications, data attributes being exchanged -
6.6.4 Summary of the testing performed on the interface to verify interoperability -
6.6.4 Description of any fault tolerance behaviour, boundary condition testing, or fail safe -
6.6.4 Known limitations (what the user should not do), contraindications, precautions and warnings -
6.6.4 Recommended connections -
IFU
Copyright © 2020 BSI. All rights reserved

55
Information to be supplied by the manufacturer (ISO 20417)
6 Requirements for accompanying information
ISO 20417:2020 EN 1041
6.6.5 If the manufacturer has a website, the IFU should be available on that website -
6.6.5 May use two-dimensional code (e.g., QR Code) as specified in ISO 22742:2010 -
6.6.5 Access instructions and how to get paper copy -
IFU
Copyright © 2020 BSI. All rights reserved
56
Information to be supplied by the manufacturer (ISO 20417)
7 Other information which is required to be supplied with the medical device
ISO 20417:2020 EN 1041
7.1 Importer name or trade name and full address -
7.2 Distributor name or trade name and full address -
7.3 Re-packager name or trade name and full address -
7.4 Translator name or trade name and full address
7.5 Regulatory identification
Other
Copyright © 2020 BSI. All rights reserved

57
Annex A (informative) Particular guidance and rationale
Annex B (informative) Example test method for assessing clearly legible
Annex C (informative) Example test method for assessing durability
Annex D (informative) Cross reference between the document and the requirements considered
Annex E (informative) Reference to the IMDRF essential principles and labelling guidances
Table E.1 — Correspondence between this document and the essential principles
Table E.2 — Correspondence between this document and the labelling principles
Annex F (informative) Reference to the essential principles
Table F.1 — Correspondence between the essential principles for non-IVD medical devices and this document
Table F.2 — Correspondence between the essential principles for IVD medical devices and this document
Annex G (informative) Reference to the general safety and performance requirements for medical devices
Annex H (informative) Reference to the general safety and performance requirements for IVD medical devices
Annex I (informative) Terminology — Alphabetized index of defined terms
Bibliography
Information to be supplied by the manufacturer (ISO 20417)
CONTENTS
Copyright © 2020 BSI. All rights reserved
58
Information to be supplied by the manufacturer (ISO 20417)
Current Status
• Final draft international standard published
• Publication remains anticipated in 2020
Copyright © 2020 BSI. All rights reserved

59
Summary
• ISO 20417:2020 expected in Q2 2020
• Significant updates to standard vs 1041
• A lot of detail and normative references
• Overlap between EU requirements already –
formalises into standard
• MDD gap analysis
Copyright © 2020 BSI. All rights reserved
